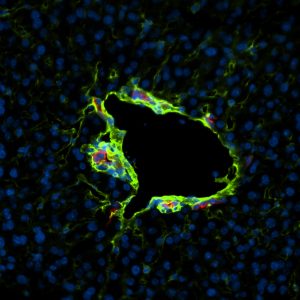
A murine liver vessel surrounded by the extracellular matrix protein laminin (green). Matrix proteins like laminin provide stability for fragile blood vessels and are susceptible to proteolytic degradation that compromises vascular integrity.
While analyzing vascular development in NuRD chromatin-remodeling complex mutants, we discovered that these mutants develop sudden and reproducible vascular rupture at midgestation due to transcriptional misregulation of genes that activate the extracellular matrix protease plasmin. Genetic reduction of plasmin activators rescued vascular matrix proteolysis and rupture in our mutants (Ingram et al, 2011). We subsequently discovered that NuRD continues to regulate plasmin activation and vascular integrity later in embryonic development, with particularly important consequences for hepatic matrix degradation and sinusoidal vascular rupture (Crosswhite et al, 2016). This work is important because it reveals a novel, chromatin-based mechanism for regulating plasmin activation. It also highlights the detrimental effects of excessive plasmin on embryonic blood vessels and thereby challenges the dogma that plasmin-mediated matrix degradation is predominantly beneficial during development because it promotes sprouting angiogenesis. We recently built upon this embryonic work to show that elevation of plasmin likewise causes matrix degradation and hepatic sinusoidal bleeding in adult mice after acetaminophen overdose (Gao et al, 2018). Altogether, our findings are generating new information about plasmin regulation and are identifying contexts in which therapeutic plasmin inhibition could protect vascular integrity. The lab was recently awarded an NIH R35 grant to expand this line of research and define how additional proteases compromise vascular integrity in development and disease.